價格:免費
更新日期:2018-04-11
檔案大小:16.2 MB
目前版本:1.1
版本需求:需要 iOS 8.0 或以上版本。與 iPhone、iPad 及 iPod touch 相容。
支援語言:英語
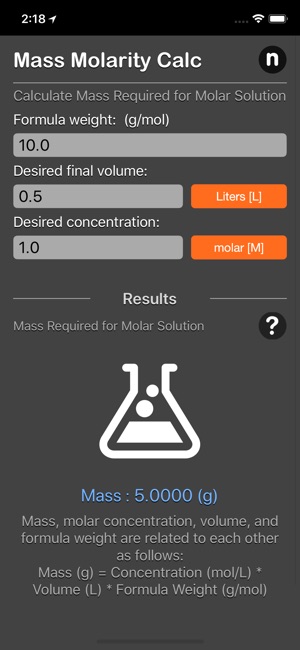
Mass molarity calculator is a useful tool which allows you to calculate the mass of a compound required to prepare a solution of known volume and concentration
An example of a molarity calculation using the mass molarity calculator
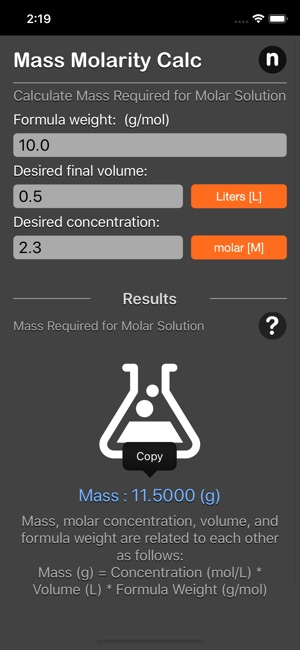
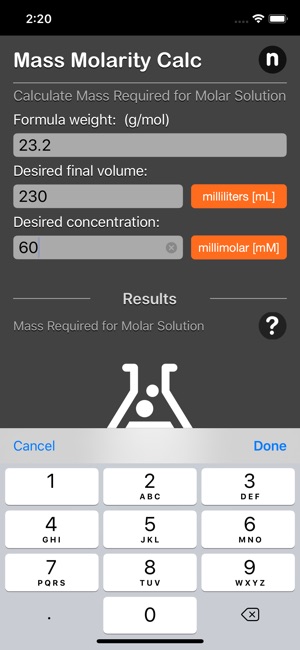
What is the mass of compound required to make a 10 mM stock solution in 10 ml of water given that the molecular weight of the compound is 197.13 g/mol?
How to calculate mass
Mass (m) is the amount of matter present in a substance. The value is constant and, unlike weight, is not affected by gravity.
Mass, molar concentration, volume, and formula weight are related to each other.
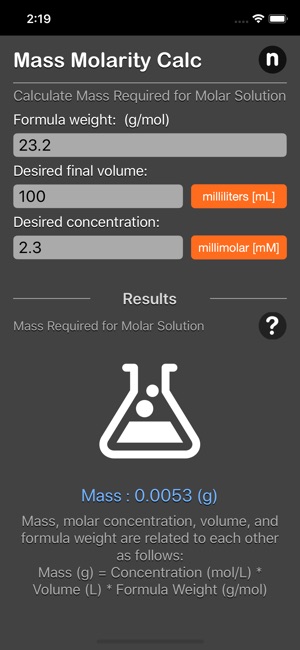
The mass molarity calculator is based on the following equation:
Mass (g) = Concentration (mol/L) * Volume (L) * Formula Weight (g/mol)
Formula weight (F.W.) is the sum of the atomic weights of all atoms in a given empirical formula. For example: Sodium chloride (NaCl) has one atom of sodium (Na) and one atom of chlorine (Cl). The atomic weight of sodium is 22.99 g/mol and chlorine is 35.45 g/mol. Therefore, the formula weight of NaCl is 58.44 g/mol (22.99 g/mol + 35.45 g/mol).
Also included chemical elements listed by atomic mass for fast reference. Table list include Atomic number, Atomic Mass, Symbol and Chemical Element name.
Molar concentration is the amount of a solute present in one unit of a solution. Its units are mol/L, mol/dm3, or mol/m3. “Molar concentration” is also known as “molarity” and can be denoted by the unit M, molar. If we want to prepare 1 L of 0.5 M sodium chloride solution, then as per the formula we require 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g).
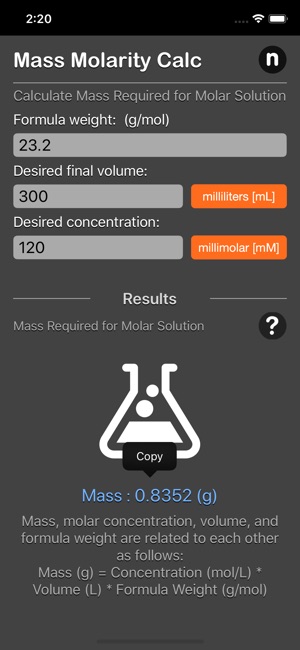
The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar concentration and volume.
*Thanks for your support, stay tune for more update to come
*This is a universal app that work for both iPhone and iPad.
支援平台:iPhone, iPad
